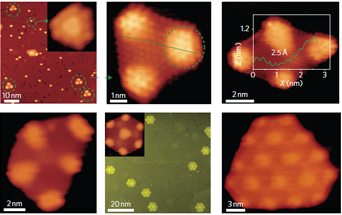
The fragmentation of fullerenes using ions, surface collisions or thermal effects is a complex process that typically leads to the formation of small carbon clusters of variable size. Here, we show that geometrically well-defined graphene quantum dots can be synthesized on a ruthenium surface using C60 molecules as a precursor. Scanning tunnelling microscopy imaging, supported by density functional theory calculations, suggests that the structures are formed through the ruthenium-catalysed cage-opening of C60. In this process, the strong C60–Ru interaction induces the formation of surface vacancies in the Ru single crystal and a subsequent embedding of C60 molecules in the surface. The fragmentation of the embedded molecules at elevated temperatures then produces carbon clusters that undergo diffusion and aggregation to form graphene quantum dots. The equilibrium shape of the graphene can be tailored by optimizing the annealing temperature and the density of the carbon clusters.
Nature Nanotechnology 6 (2011) 247.